Roman Serrat, Giovanni Marsicano, Sandrine Pouvreau et al in Cell Reports
Stars and power plants: Mitochondrial CB1 receptors regulate calcium dynamics in astrocytes
Commentary
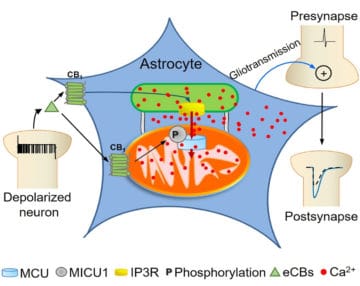
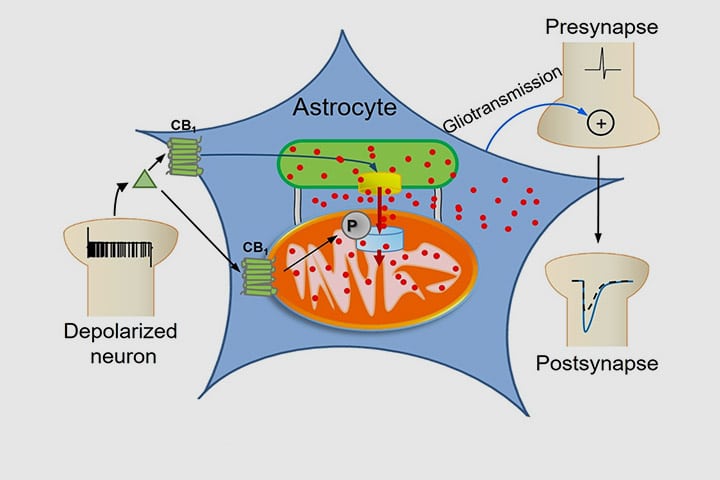
Astrocytes are key players in the regulation of synaptic transmission and plasticity. Detection of synaptic activity by astrocytes and subsequent release of gliotransmitters involve changes in astroglial Ca2+ signalling. However, how increases of Ca2+ in these cells translate into specific actions to modulate neuronal functions is largely unknown. This study shows a fully new mechanism linking the activation of CB1 receptors to the ability of mitochondria to regulate the dynamics of intracellular Ca2+.
The study represents the first outcome of a long-lasting collaboration between Giovanni Marsicano and Sandrine Pouvreau (formerly Mulle’s lab member) initiated thanks to the LabEx BRAIN CANNACALC that provided salary for Román Serrat who was a researcher in the laboratory of Giovanni Marsicano at the NeuroCentre Magendie and is now researcher at Aelis Farma. Moreover, the project greatly benefited from the collaboration with teams at the NeuroCentre Magendie (Beyeler) and IINS (Gambino).
Abstract
Intracellular calcium signaling underlies the astroglial control of synaptic transmission and plasticity. Mitochondria-endoplasmic reticulum contacts (MERCs) are key determinants of calcium dynamics, but their functional impact on astroglial regulation of brain information processing is currently unexplored. We found that the activation of astrocyte mitochondrial-associated CB1 receptors (mtCB1) determines MERCs-dependent intracellular calcium signaling and synaptic integration. The stimulation of mtCB1 receptors promotes calcium transfer from the endoplasmic reticulum to mitochondria through a specific molecular cascade, involving the mitochondrial calcium uniporter (MCU). Physiologically, mtCB1-dependent mitochondrial calcium uptake determines the dynamics of cytosolic calcium events in astrocytes upon endocannabinoid mobilization. Accordingly, electrophysiological recordings in hippocampal slices showed that conditional genetic exclusion of mtCB1 receptors or dominant negative MCU expression in astrocytes blocks lateral synaptic potentiation, through which astrocytes integrate the activity of distant synapses. Altogether, these data reveal an endocannabinoid link between astroglial MERCs and the regulation of brain network functions.
Article
Astroglial ER-mitochondria calcium transfer mediates endocannabinoid-dependent synaptic integration
Roman Serrat, Ana Covelo, Vladimir Kouskoff, Sebastien Delcasso , Andrea Ruiz-Calvo, Nicolas Chenouard, Carol Stella, Corinne Blancard, Benedicte Salin, Francisca Julio-Kalajzić, Astrid Cannich, Federico Massa, Marjorie Varilh, Severine Deforges, Laurie M Robin, Diego De Stefani, Arnau Busquets-Garcia, Frederic Gambino, Anna Beyeler, Sandrine Pouvreau, Giovanni Marsicano
Cell Rep. 2021 Dec 21;37(12):110133.
10.1016/j.celrep.2021.110133
Contact
Last update 17/01/22
