Regulation of different phases of AMPA receptor intracellular transport by 4.1N and SAP97
The modulation of the efficiency of synaptic transmission between neurons is one of the fundamental processes of memory and learning phenomena. This regulation of the strength of synaptic transmission is largely driven by changes in the number of receptors present at the synapse. In this work, the researchers identify a new mechanism for controlling the establishment of receptors at the level of the synapse through the control of their intracellular transport.
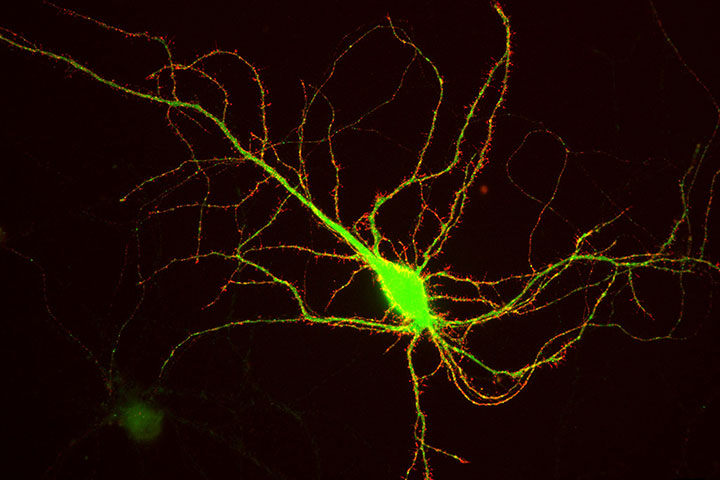
Neurotransmitter receptors, and in particular glutamate receptors, are concentrated in synapses in front of neurotransmitter release sites. However, in the process of their biogenesis, these receptors are synthesized at the level of the endoplasmic reticulum, most of the time several hundred microns from the synapses. They must therefore be transported to the synapses. Our previous work had made possible to visualize for the first time the intracellular transport of AMPA-type glutamate receptors, responsible for the majority of the rapid excitatory transmission between neurons. These receptors are transported rapidly (1-2 microns per second) in vesicles circulating on the microtubules using molecular motors. We observed that, surprisingly, this transport was strongly regulated by neuronal activity.
In this new work, we have identified the molecular mechanisms responsible for these regulations. The cytosolic C-terminal domain of the AMPAR GluA1 subunit is specifically associated with two proteins, 4.1 N and SAP97. We analyzed how interactions between GluA1 and 4.1N or SAP97 regulate GluA1 transport and its exocytosis under basal conditions and after induction of synaptic plasticity (LTP). Our results identify differential roles of 4.1N and SAP97 in controlling the different phases of transport and membrane integration of GluA1.
This work opens new perspectives in understanding the molecular mechanisms that control the establishment and maintenance of glutamate receptors at the synapse during synaptic plasticity.
Françoise Coussen, Director of research at the CNRS at IINS worked on AMPAR intracellular transport and directed this work helped by Daniel Choquet. Caroline Bonnet, PhD student, performed the experiments helped by Justine Charpentier who performed all biochemistry experiments and by Natacha Retailleau (molecular biology).
Article and authors information
Regulation of different phases of AMPA receptor intracellular transport by 4.1N and SAP97
Caroline Bonnet, Justine Charpentier, Natacha Retailleau, Daniel Choquet, Françoise Coussen-Choquet
eLife. 2023 April 20. DOI : 10.7554/eLife.85609
Contact
Françoise Coussen-Choquet
Chercheur (DR) / CNRS / IINS
+33 5 33 51 47 34
Last update 25/04/23
