G. Giannone et al in Nature Cell Biology
Voir le communiqué en français
Abstract
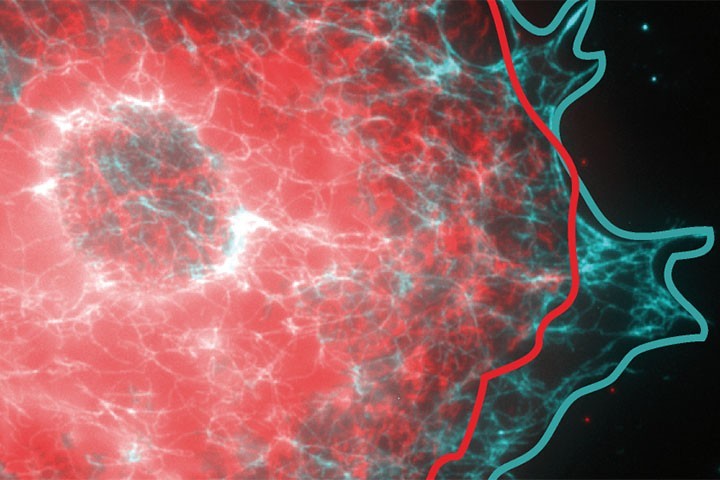
Detection and conversion of mechanical forces into biochemical signals controls cell functions during physiological and pathological processes. Mechanosensing is based on protein deformations and reorganizations, yet the molecular mechanisms are still unclear. Using a cell-stretching device compatible with super-resolution microscopy and single-protein tracking, we explored the nanoscale deformations and reorganizations of individual proteins inside mechanosensitive structures. We achieved super-resolution microscopy after live stretching on intermediate filaments, microtubules and integrin adhesions. Simultaneous single-protein tracking and stretching showed that while integrins followed the elastic deformation of the substrate, actin filaments and talin also displayed lagged and transient inelastic responses associated with active acto-myosin remodelling and talin deformations. Capturing acute reorganizations of single molecules during stretching showed that force-dependent vinculin recruitment is delayed and depends on the maturation of integrin adhesions. Thus, cells respond to external forces by amplifying transiently and locally cytoskeleton displacements, enabling protein deformation and recruitment in mechanosensitive structures.
Reference
Sophie Massou*, Filipe Nunes Vicente*, Franziska Wetzel*, Amine Mehidi, Dan Strehle, Cecile Leduc, Raphaël Voituriez, Olivier Rossier, Pierre, Nassoy and Gregory Giannone
Cell stretching is amplified by active actin remodeling to deform and recruit proteins in mechano-sensitive structures.
Nature Cell Biology
DOI : 10.1038/s41556-020-0548-2.
https://www.nature.com/articles/s41556-020-0548-2
* co first authors
Contacts
Grégory Giannone
Univ. Bordeaux, CNRS, Institut Interdisciplinaire de Neurosciences, UMR 5297
Email:
Pierre Nassoy
Univ. Bordeaux, CNRS & Institut d’Optique, LP2N
Email:
Last update 31/07/20
