Etienne Herzog in eLife
A proline-rich motif on VGLUT1 reduces synaptic vesicle super-pool and spontaneous release frequency.
“Should I stay or should I go” may summarize the mechanisms at play acting on synaptic vesicles to cluster them at synapses or push them to other synapses of the neuron. In neuronal networks, information flows along neuronal membranes through electric waves and between neurons mostly through chemical neurotransmission. This elementary event of information transfer in the nervous system supports from the simplest unconscious tasks of brain functions to the most complex motivated behaviors or autonomic regulations of body physiology. Efficient neurotransmission requires 5 tightly regulated steps. Step 1 is the synthesis of the neurotransmitter. Step 2 is the accumulation of neurotransmitter molecules in synaptic vesicles. During step 3 synaptic vesicles ready for secretion are fusing with the neuron membrane upon stimulation by an electric wave (called “action potential”). Chemical neurotransmission occurs at step 4 when neurotransmitter molecules secreted in the synaptic cleft, bind to specific receptors that transmit new electric waves and intracellular signaling in the targeted neurons. Neurotransmission ends at step 5 when specific mechanisms destroy or remove the neurotransmitter molecules from the synaptic cleft. The tight pacing of neurotransmission with the occurrence of action potentials is a key element for normal brain function. Our work focuses on step 3 when neurotransmitter-filled synaptic vesicles are maintained in a dynamic equilibrium between a synaptic cluster of vesicles where they may undergo maturation for release, and the transport flow responsible for the distribution of vesicles to all synapses of the neuron. Synaptic vesicles are composed of lipids and proteins. Our work shows that adding a specific stretch of amino-acids (proline rich domain) to a synaptic vesicle protein sequence is sufficient to displace the equilibrium towards synaptic vesicles cluster stabilization with a consecutive reduction on synaptic vesicle release rates. We also show that the glutamate transporter (VGLUT1) responsible for filling synaptic vesicles during step 2 of neurotransmission acquired such proline-rich sequence during the evolution of mammals. This gain of function leads to a transporter that tune both the level of glutamate inside synaptic vesicles and the supply of synaptic vesicles for glutamate release during excitatory neurotransmission. Our findings shed light on the importance of weak protein-protein interactions mediated by proline-rich domains in tuning synaptic vesicle mobility stages and neurotransmission. Future work should investigate whether this tuning may be regulated during synaptic plasticity and associated learning.
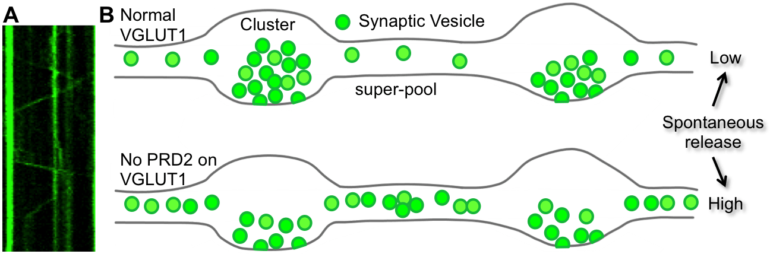
Figure: VGLUT1 reduces synaptic vesicle super-pool and spontaneous glutamate release. A: Example of super-pool vesicle mobility analysis using a kymograph. B: Schematic representation of proline rich domain 2 sequence effect on axonal synaptic vesicle homeostasis and spontaneous glutamate release. Copyrights : Etienne HERZOG
Zhang XM, Francois U, Silm K, et al., (2019) A proline-rich motif on VGLUT1 reduces synaptic vesicle super-pool and spontaneous release frequency. eLife 8:e50401
https://doi.org/10.7554/eLife.50401
Correspondance :
Last update 04/12/19

 Dr Etienne HERZOG
Dr Etienne HERZOG