David Perrais and Matthieu Sainlos in Nature Communications
Abstract
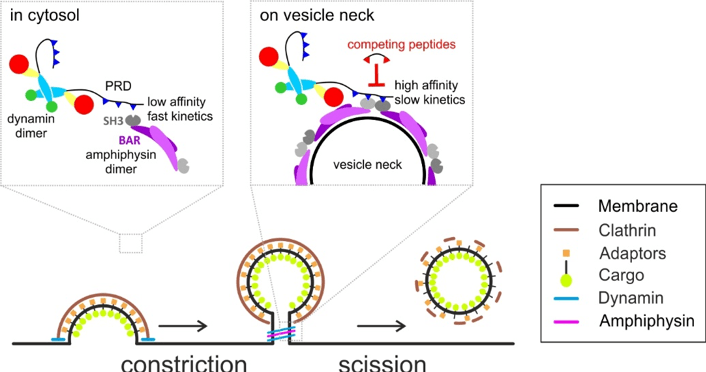
Endocytosis, the formation of vesicles from the plasma membrane, is an essential process in cells. It is involved for example in the local recycling of synaptic vesicles, basic components of synapses in the brain. Endocytic vesicles are created by the successive action of many proteins that deform the plasma membrane into a bud and finally cut the membrane. This last step is performed by the GTPase dynamin, recruited to the neck of the forming vesicle by other proteins which interact with dynamin. However, these interactions are labile and with many different proteins. Therefore, it is still unclear how dynamin is recruited efficiently at the last step of vesicle formation, and hence how it can be modulated or blocked. David Perrais, Matthieu Sainlos and collaborators in the team of Daniel Choquet have combined live cell fluorescent imaging of endocytosis and peptide chemistry to design new tools to understand and interfere specifically with endocytosis. They have found that dynamin must interact simultaneously with two molecules of the associated protein amphiphysin to be recruited. This can only occur when amphiphysin assembles around the neck of the forming vesicle, providing an elegant explanation of the fast and timely recruitment of dynamin (Figure). On the way, they have designed new highly specific and efficient multivalent peptides to block endocytosis. These new tools will be useful to address the role of endocytosis in various cellular processes like synaptic transmission and plasticity, cell motility or viral entry.
Last update 16/10/19
