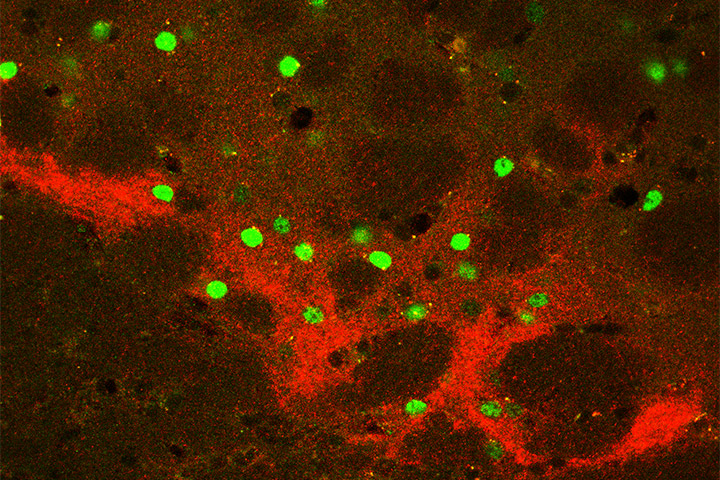
New mechanism for an old relationship between striatopallidal CB1 receptor and amphetamine sensitization.
Psychostimulants drugs like amphetamine directly enhance dopaminergic transmission. As a consequence, they temporarily increase movement, mental alertness, and attention inducing a sense of euphoria in humans, and trigger hyperlocomotion and stereotypies in rodents. The repeated exposure to psychostimulants (e.g., amphetamine) causes a long-lasting enhancement in the behavioral responses to the same dose of the drug, called behavioral sensitization. The neuronal changes accompanying sensitization have been hypothesized to, at least partially, underlie abuse and addiction to psychostimulants. This phenomenon involves several neuronal systems and brain areas, however, the mechanisms regulating the initiation and expression of behavioral sensitization are still under investigation.
The dorsal striatum has been identified as a key region for the motor-related effects of psychostimulants. In particular, amphetamine injections have been shown to induce an increase of the dopaminergic tone within the dorsal striatum, resulting in hyperlocomotion through the modulation of the activity of the main cell type of this region, the medium spiny neurons (MSNs). Both striatonigral neurons (i.e., direct pathway MSNs (dMSNs), mainly characterized by the expression of the D1 dopamine receptor) and striatopallidal neurons (i.e., indirect pathway MSNs (iMSNs), identified by the expression of the D2 dopamine receptor) have been shown to participate in the behavioral sensitization induced by amphetamine. While the transient blockade of the dMSNs was found to impair the expression of behavioral sensitization, the inhibition of iMSNs enhanced this behavior.
The endocannabinoid system (ECS) has been proposed to be involved in the response to psychostimulants and, particularly, in the expression of behavioral sensitization. Indeed, this phenomenon is accompanied by increased endocannabinoid tone, and genetic or pharmacological manipulations of ECS activity were shown to modulate the expression of psychostimulants-induced sensitization. However, the neuronal basis of this interaction has not been investigated. In the CNS, the ECS exerts its functions mainly acting through the cannabinoid type-1 (CB1) receptor, which is highly expressed at terminals of striatal MSNs belonging to direct and indirect pathways.
Therefore, this study aimed at identifying the involvement of striatal CB1 receptors in different striatal subpopulations in the expression of behavioral sensitization to amphetamine, and in the modulation of related functional changes induced by this drug of abuse in mice. We show that, while striatal CB1 receptors are not involved in the acute response to amphetamine, the behavioral sensitization requires the activation of CB1 receptors specifically located at striatopallidal MSNs (indirect pathway). Accordingly, we found that repeated treatment with amphetamine promotes functional changes that do not manifest in animals lacking CB1 receptors in striatopallidal MSNs. Finally, we show that repeated amphetamine administration induces hypersensitivity to the drug by reducing striatopallidal transmission, and this effect is mediated by CB1 in this striatal population. These results highlight a new mechanism of psychostimulant sensitization, a phenomenon that plays a key role in the health-threatening effects of these drugs.
This research work was led by Giovanni Marsicano and Luigi Bellocchio, in the “Endocannabinoids and Neuroadaptation” team at the Neurocentre Magendie. For the success of this work, the two researchers collaborated with Jérôme Baufreton’s team at the Institute of Neurodegenerative Diseases in Bordeaux.
Reference
Striatopallidal cannabinoid type-1 receptors mediate amphetamine-induced sensitization
Yamuna Mariani 1 , Ana Covelo 1 , Rui S Rodrigues 1 , Francisca Julio-Kalajzić 1 , Antonio C Pagano Zottola 2 , Gianluca Lavanco 3 , Michela Fabrizio 4 , Doriane Gisquet 1 , Filippo Drago 5 , Astrid Cannich 1 , Jerome Baufreton 6 , Giovanni Marsicano 7 , Luigi Bellocchio 8
1 Univ. Bordeaux, INSERM, Neurocentre Magendie, U1215, 33000 Bordeaux, France.
2 Univ. Bordeaux, INSERM, Neurocentre Magendie, U1215, 33000 Bordeaux, France; Institut de Biochimie et Génétique Cellulaires, UMR 5095, 33077 Bordeaux, France.
3 Univ. Bordeaux, INSERM, Neurocentre Magendie, U1215, 33000 Bordeaux, France; University of Palermo, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties of Excellence “G. D’Alessandro,” 90127 Palermo, Italy.
4 Univ. Bordeaux, INSERM, Neurocentre Magendie, U1215, 33000 Bordeaux, France; Center for Interdisciplinary Research in Biology (CIRB), College de France, CNRS, INSERM, 5 Université PSL, 75231 Paris, France.
5 Department of Biomedical and Biotechnological Sciences, Section of Pharmacology, University of Catania, Catania 95124, Italy.
6 Univ. Bordeaux, CNRS, IMN, UMR 5293, 33000 Bordeaux, France.
7 Univ. Bordeaux, INSERM, Neurocentre Magendie, U1215, 33000 Bordeaux, France. Electronic address: .
8 Univ. Bordeaux, INSERM, Neurocentre Magendie, U1215, 33000 Bordeaux, France. Electronic address: .
October 24, 2023
Current Biology
DOI:https://doi.org/10.1016/j.cub.2023.09.075
Last update 23/11/23
