Wilfrid Mazier (Equipe Cota) & al. dans Molecular Metabolism
https://doi.org/10.1016/j.molmet.2019.08.005
*Corresponding author. Group“Energy Balance and Obesity”, INSERM U1215, Neurocentre Magendie, 146 Rue Léo Saignat, 33077 Bordeaux, France. E-mail:
Abstract
Objective
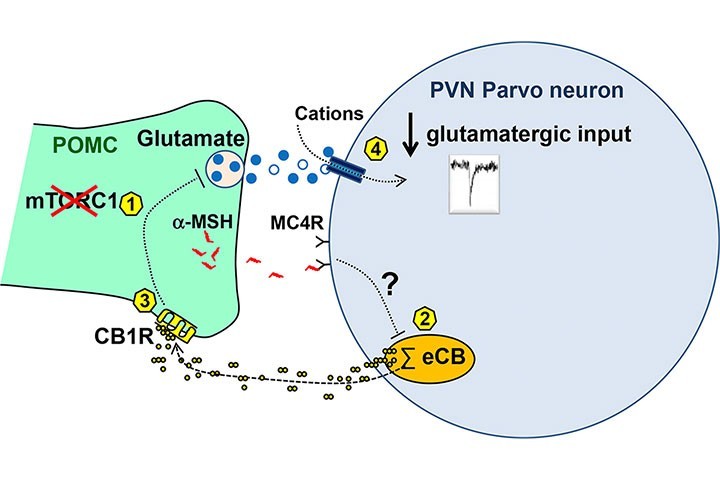
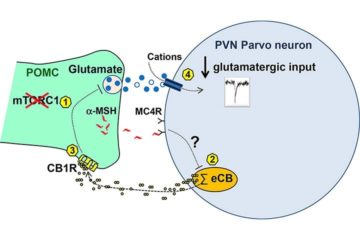
The hypothalamic paraventricular nucleus (PVN) is a key target of the melanocortin system, which orchestrates behavioral and metabolic responses depending on energy availability. The mechanistic target of rapamycin complex 1 (mTORC1) and the endocannabinoid type 1 receptor (CB1R) pathways are two key signaling systems involved in the regulation of energy balance whose activity closely depends upon energy availability. Here we tested the hypothesis that modulation of mTORC1 and CB1R signaling regulates excitatory glutamatergic inputs onto the PVN.
Methods
Patch-clamp recordings in C57BL/6J mice, in mice lacking the mTORC1 component Rptor or CB1R in pro-opio-melanocortin (POMC) neurons, combined with pharmacology targeting mTORC1, the melanocortin receptor type 4 (MC4R), or the endocannabinoid system under chow or a hypercaloric diet.
Results
Acute pharmacological inhibition of mTORC1 in C57BL/6J mice decreased glutamatergic inputs onto the PVN via a mechanism requiring modulation of MC4R, endocannabinoid 2-AG mobilization by PVN parvocellular neurons, and retrograde activation of presynaptic CB1R. Further electrophysiology studies using mice lacking mTORC1 activity or CB1R in POMC neurons indicated that the observed effects involved mTORC1 and CB1R-dependent regulation of glutamate release from POMC neurons. Finally, energy surfeit caused by hypercaloric high-fat diet feeding, rapidly and time-dependently altered the glutamatergic inputs onto parvocellular neurons and the ability of mTORC1 and CB1R signaling to modulate such excitatory activity.
Conclusions
These findings pinpoint the relationship between mTORC1 and endocannabinoid-CB1R signaling in the regulation of the POMC-mediated glutamatergic inputs onto PVN parvocellular neurons and its rapid alteration in conditions favoring the development of obesity.
Comment by Daniela Cota :
Mise à jour: 22/10/19
