Sandra Soukup in Neuron
A novel EndophilinA Parkinson’s risk mutation blocks waste recycling via autophagy at the synapse
Macroautophagy (hereafter referred to as autophagy) is a conserved pathway across evolution in which cytoplasmic material such as proteins, lipids and organelles are engulfed into a growing membrane named autophagosome that in turn fuses with the lysosome giving rise to the autolysosome where degradation occurs. Autophagy plays a critical role in neuronal function and survival. Intriguingly, although emerging evidence supports the role of autophagy in synaptic function, the precise molecular mechanisms linking neuronal activity, autophagy and disease were vastly unknown.
Now an international and multidisciplinary collaboration between the Bordeaux Neurocampus members Sandra Soukup (IMN and co-corresponding author of the article) and Jean-Baptiste Sibarita (IINS) together with the laboratory of Patrik Verstreken in Leuven (director of VIB-KU Leuven Center for Brain & Disease Research and co-corresponding author) sheds light on this problem by reporting in Neuron how neuronal activity regulates synaptic autophagy via calcium influx and the synaptic protein EndophilinA. Moreover, the authors report for the first time how a novel mutation in EndophilinA that increases the risk of Parkinson’s disease (PD) causes the disruption of synaptic autophagy in response to calcium influx leading to neurodegeneration in a process that is evolutionarily conserved between flies and humans.
Amino acid deprivation (a.k.a. starvation) induces autophagosome and autolysosome formation at the presynaptic compartment of Drosophila neurons. Previous work of Dr. Soukup showed that neuronal activity can act as a trigger of autophagy at the synapse (Soukup et al., Neuron 2016). Still, the molecular mechanisms of this pathway were unknown. In this article, we decipher how extracellular Ca2+influx into the presynaptic terminal constitutes the initial stimulus for autophagosome formation at the synapse in response to neuronal activity, likely acting as a rapid mechanism to support synaptic homeostasis during periods of intense neuronal stimulation.
Previous work from Dr. Soukup shows that the synaptic protein EndophilinA plays a fundamental role in the process of autophagosome biogenesis at the synapse in response to both neuronal activities but also starvation. We now combine hydrogen-deuterium exchange mass spectrometry (HDX-MS) and single particle tracking PALM (sptPALM) to show how a residue (D265) in this protein dictates the switch from cytoplasmic to autophagic membranes in response to calcium influx, providing mechanistic evidence of how neuronal activity can control autophagosome biogenesis at the synapse.
Strikingly, this highly collaborative and multidisciplinary effort in fundamental research served us to further investigate the role of EndophilinA and synaptic autophagy in neurodegenerative diseases. We further demonstrate how a recently reported PD-linked mutation (G276V) in the human EndophilinA1 (SH3GL2) blocks synaptic autophagy. Moreover, we discovered that this PD risk mutation disrupts the calcium-sensing of EndophilinA1, leading to an immobile protein that cannot respond to calcium influx and disrupting autophagy in dopaminergic neurons. Furthermore, we showed that calcium-mediated autophagy induction via EndophilinA was critical for neuronal survival.
A comprehensive characterization of the molecular pathways and intermediates functioning in autophagy at different neuronal compartments emerges as an essential task to develop promising therapeutic opportunities targeting autophagy to treat neurodegenerative diseases. In this context, our work provides valuable information regarding the stimuli and intermediates functioning in synaptic autophagy in Parkinson’s disease.
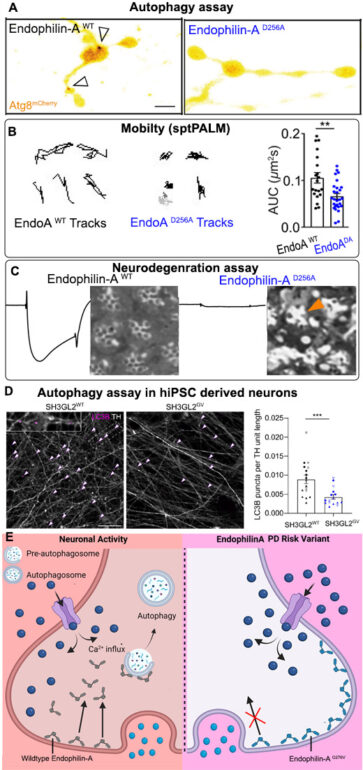
Figure legend
A) Autophagy assay in Drosophila presynaptic terminals shows autophagosomal formation (arrows, Atg8 positive structures) upon induction of neuronal activity. Synapses expressing EndophilinA with the calcium-insensitive mutation D256A fail to form autophagosomes in response to neuronal activity.
B) Single particle tracking (sptPALM) reveals calcium-insensitive Endophilin-A is less mobile than wild-type EndophilinA.
C) Neurodegeneration assay in Drosophila photoreceptors showing electroretinograms (ERGs) recordings to measure photoreceptor activity and histological sections to analyse photoreceptor morphology. Calcium-insensitive EndophilinA mutant shows greatly reduced photoreceptor activity and photoreceptor neurodegeneration (arrows) compared to wild-type animals where photoreceptor activity and morphology are normal.
D) Dopaminergic neurons differentiated from human induced pluripotent stem cells (hiPSC) genetically engineered with the Parkinson risk mutation (G276V) in SH3GL2 (EndophilinA1) show fewer autophagosomes (arrows, LC3 positive structures) than dopaminergic neurons derived from hiPSC harboring wild type SH3GL2.
E) Graphical summary
Reference
EndophilinA-dependent coupling between activity-dependent calcium influx and synaptic autophagy is disrupted by a Parkinson-risk mutation. Bademosi A.T, Decet M, Kuenen S, Calatayud C, Swerts J, Gallego S.F, Schoovaerts N, Louros N, Martin E, Karamanou S, Sibarita JB, Vints K, Gounko N.V, Meunier F.A, Economou A, Versees W, Rousseau F, Schymkowitz J, Soukup SF, Verstreken P. Neuron 111, 1–21 May 3, 2023;
doi: 10.1016/j.neuron.2023.02.001
Contact
Sandra Soukup
Team Molecular Mechanisms of Synaptopathies
IMN (Institut des maladies neurodégénératives)
Mise à jour: 21/03/23