Roman Walle, Pierre Trifilieff et al. in Nature Communications
Nucleus accumbens D1- and D2-expressing neurons control the balance between feeding and activity-mediated energy expenditure
Article in collaboration with IMN (François Georges, Giulia Fois), Neurocentre Magendie (Francis Chaouloff), McGill Douglas Research Center (Bruno Giros), Université Libre de Bruxelles (Alban de Kerchove d’Exaerde, Christophe Varin), UMC Utrecht (Roger A. Adan).
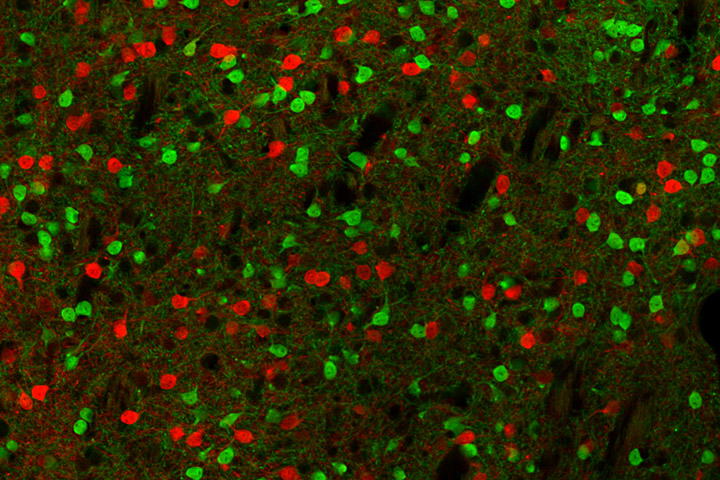
Overeating/obesity-related pathologies and anorexia nervosa (AN) are multifactorial diseases resulting from an interaction between genetic and environmental factors. These eating disorders (ED) are characterized by psychiatric symptoms coupled to metabolic alterations, making them increasingly viewed as “metabo-psychiatric” diseases. Dimensional approach to EDs reveals alterations in common domains such as negative (stress, anxiety) and positive (motivation, reward processing) valence systems. In particular, beyond the opposite feeding patterns, which might be related to inverse alterations of food-related reward valuation, reward processing tends to be dysregulated in opposite manners in overeating and AN. In particular, an increased drive to exercise due to enhanced reinforcing value of physical activity is a typical feature of AN that persists after remission and might even precede the development of the disease. On the other hand, obesity is associated with physical hypoactivity even after recovery. These differential alterations of physical activity in EDs have been proposed to be linked to the rewarding impact of exercise and/or the motivation to engage in physical activity. Such overlapping symptomatic dimensions in EDs suggest opposite dysfunctions within common brain networks, especially the reward system. In accordance, functional imaging reveals that activity of the Nucleus Accumbens (NAc) is regulated in opposite manner in AN and obese subjects. However, to which extent dysfunction of the NAc is sufficient to elicit ED-like behavioral phenotypes is not well understood.
Using a chemogenetic approach we found that activity of dopamine D1 receptor-expressing neurons of the NAc core subregion facilitated effort for a food reward as well as voluntary exercise, but decreased food intake, while D2-expressing neurons have opposite effects. These findings are congruent with D2-neurons being more active than D1-neurons during feeding while it is the opposite during running, as evaluated by calcium imaging through fiber photometry recording. Chronic chemogenetic manipulations of each subpopulations had limited effects on the balance between food intake and activity-mediated energy expenditure. Thanks to a novel transgenic mouse model developed by our collaborators B. Giros and A. de Kerchove d’Exaerde, we manipulated both neuronal subpopulations concomitantly. Repeated activation of D1-neurons combined with inhibition of D2-neurons biased behavior toward activity-related energy expenditure, whilst the opposite manipulations favored energy intake. Strikingly, concomitant activation of D1-neurons and inhibition of D2-neurons precipitated weight loss in rodent anorexia models due to exaggerated physical activity.
These observations support that, under physiological conditions, the recruitment of the NAc network in favor of D1-neurons would bias behavior towards effort exertion – including food-seeking – at the expense of food consumption. In opposite, a predominant activity of D2-neurons would blunt voluntary activity whilst promoting food intake. Such a pivotal role of the NAc neuronal network is in accordance with the implication of the NAc as a “sensory sentinel”, allowing rapid control over food consumption in response to motivational or sensory signals. From a translational prospective, these results suggest that a chronic imbalance in the NAc neuronal network might be at the core of EDs by altering homeostatic balance.
Reference
Nucleus accumbens D1- and D2-expressing neurons control the balance between feeding and activity-mediated energy expenditure.
Walle R, Petitbon A, Fois GR, Varin C, Montalban E, Hardt L, Contini A, Angelo MF, Potier M, Ortole R, Oummadi A, De Smedt-Peyrusse V, Adan RA, Giros B, Chaouloff F, Ferreira G, de Kerchove d’Exaerde A, Ducrocq F, Georges F, Trifilieff P.
Nat Commun. 2024 Mar 21;15(1):2543.
doi : 10.1038/s41467-024-46874-9.
First Author
 Roman Walle completed his PhD under the supervision of Pierre Trifilieff. During his thesis, he focused on the role of dopaminoceptive neuronal subpopulations of the ventral striatum in the control of incentive processes, food intake and voluntary exercise.
Roman Walle completed his PhD under the supervision of Pierre Trifilieff. During his thesis, he focused on the role of dopaminoceptive neuronal subpopulations of the ventral striatum in the control of incentive processes, food intake and voluntary exercise.
Roman is now a postdoctoral researcher in Francesco Papaleo’s lab in the Istituto Italiano di Tecnologia (IIT) in Genova where he develops a project regarding the role of noradrenergic projections to the medial Prefrontal cortex on natural socio-affective behaviors. By using optogenetic, fiberphotometry and calcium imaging approaches, his project assesses the relative importance of the noradrenergic transmission in the excitation/inhibition balance between pyramidal and somatostatin interneurons.
Mise à jour: 23/04/24
