
When a Synthetic Fibril Self‑Replicates in the Brain
Multiple system atrophy (MSA) is a rare, incurable neurodegenerative disease marked by intracellular inclusions composed of alpha‑synuclein fibrils. In a paper published in Nature, scientists show that a particular model of alpha‑synuclein fibril created artificially in vitro can self‑replicate and proliferate in the mouse brain, recapitulating the intracerebral inclusions seen in the human disease. These results open new avenues for understanding prion‑like mechanisms and for designing novel inhibitors.
An experiment to understand how pathogenic fibrils spread in the brain
Multiple system atrophy (MSA) is a rare and severe neurodegenerative disease caused by the massive, rapid accumulation of α‑synuclein fibrils in the brain. This accumulation is also observed in other synucleinopathies such as Parkinson’s disease and dementia with Lewy bodies, but with a much slower rate of progression. The key question that remains is: what gives certain fibrils the ability to spread with the speed of infectious agents, while others evolve much more slowly over many years?
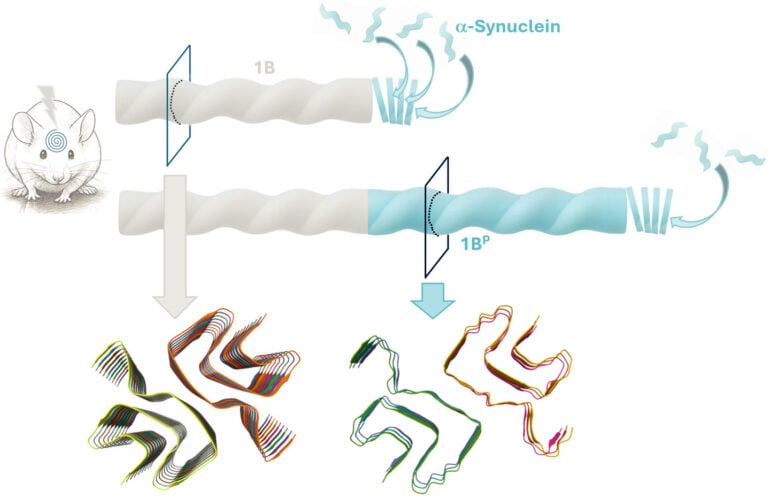
To address this, scientists created in the laboratory a specific α‑synuclein fibril, dubbed 1B, and injected it into the brains of mice. This synthetic entity very rapidly induced pathological inclusions similar to those observed in MSA. The results of this study, published in Nature, shed new light on the mechanisms by which synucleinopathies propagate.
Self‑replication observed at atomic resolution
Using cryo‑electron microscopy, which makes it possible to study protein structures at atomic scale, the scientists examined the synthetic fibrils before inoculation (1B) and those produced in the brain as a result of inoculation (1BP). The two structures proved to be almost identical. 1BP retains the same folding, pairing and stacking architecture as 1B. This similarity demonstrates that the synthetic 1B fibril generated its own copy in the organism—a process that amounts to genuine in vivo self‑replication. The existence of such a phenomenon had never previously been demonstrated at atomic resolution in an animal, not even for prions. Moreover, diluted mouse‑brain homogenates containing these 1BP fibrils can in turn transmit the pathology to other animals upon reinjection.
Toward a better understanding and new therapeutic strategies
The scientists also identified specific structural regions that appear to play a central role in these fibrils’ ability to multiply and to evade cellular degradation systems.
“These findings provide experimental proof that a conformational, prion‑like replicative mechanism is at work in synucleinopathies,” notes François Ichas. “They open avenues for understanding how certain forms of α‑synuclein assembly become pathogenic and for designing strategies to interrupt this process.”
This work provides a robust experimental model of the prion‑like mechanisms underlying MSA and other synucleinopathies, such as Parkinson’s disease and dementia with Lewy bodies. It also highlights the supramolecular structural underpinnings that differentiate these disorders. In the longer term, identifying the critical interfaces exposed by 1B fibrils could guide the design of inhibitors capable of preventing their spread. This discovery also invites a rethinking of the boundaries between biological entities and artificially derived pathogenic agents.
Reference
Synthetic α-synuclein fibrils replicate in mice causing MSA-like pathology
Domenic Burger, Marianna Kashyrina, Lukas van den Heuvel, Hortense de La Seiglière, Amanda J. Lewis, Francesco De Nuccio, Inayathulla Mohammed, Jérémy Verchère, Cécile Feuillie, Mélanie Berbon, Marie-Laure Arotcarena, Aude Retailleau, Erwan Bezard, Marie-Hélène Canron, Wassilios G. Meissner, Antoine Loquet, Luc Bousset, Christel Poujol, K. Peter, R. Nilsson, Florent Laferrière, Thierry Baron, Dario Domenico Lofrumento, Francesca De Giorgi, Henning Stahlberg, François Ichas
https://www.nature.com/articles/s41586-025-09698-1
Contacts
Dr. François Ichas
Institut des Maladies Neurodégénératives CNRS Université de Bordeaux, France
Laboratoire d’Anatomie Humaine, DiSTeBA, Université du Salento, Lecce, Italie.
Prof. Henning Stahlberg,
Laboratoire de Microscopie Electronique Appliquée à la Biologie, Ecole Polytechnique Fédérale de Lausanne, Université de Lausanne, Lausanne, Suisse.
Last update 05/11/25
